Abstract
Background: Infectious disease plays a central role in malignancy, with up to one in six cancers having a microbial association (Parkin Int. J. Cancer 2006). Lymphomas in particular are associated with multiple viral pathogens, including Epstein Barr virus (EBV), Kaposi Sarcoma herpesvirus (KSHV), and HIV. Sequencing of cell-free DNA (cfDNA) is an emerging technique in the diagnosis and surveillance of cancer. While studies to date have focused primarily on tumor-associated somatic variants, cfDNA may also provide insight into the infectious and immune state of cancer patients. We examined cfDNA from lymphoma patients of multiple histologic subtypes to characterize viral detection and dynamics.
Methods: Plasma from 360 pre-treatment patients with various lymphoma histologies was analyzed along with that of 69 healthy adults. Multiple samples per patient were included when available. All samples underwent deep sequencing with error correction by CAPP-Seq (Newman Nat Biotech 2016). Reads were filtered for homology to the human genome and endogenous retroviruses, mapped to NCBI consensus genomes for human-hosted viral species, and filtered by breadth of genomic coverage. Viral read count was normalized by total sequencing depth to determine viral read fraction (VRF). EBV fragment size was assessed via single-read BLAST alignment length considering reads with expect value < 1E-5. Integration sites were assessed with the VirusClip package (Ho Oncotarget 2015).
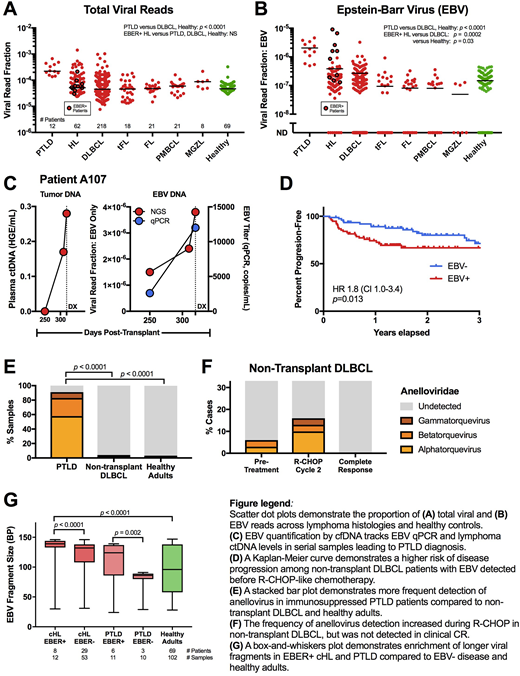
Results: Patients with most lymphoma histologic subtypes had viral loads not significantly different from those of healthy adults. However, post-transplant lymphoproliferative disorder (PTLD) patients receiving immunosuppression for solid organ transplants had significantly increased total viremia (Fig 1A) and EBV levels (Fig 1B) when compared to healthy adults and non-transplant DLBCL patients. EBER+ classical Hodgkin lymphoma (cHL) displayed no difference in total viremia but had significantly elevated EBV. In an EBV-positive PTLD patient, cfDNA viral levels tracked both clinical viral qPCR and circulating tumor DNA (ctDNA) levels in serial samples leading to diagnosis (Fig 1C).
Elevated EBV levels were also present in a subset of non-transplant DLBCL. In a cohort of DLBCL patients treated with frontline R-CHOP-like chemotherapy (n=152), individuals with pre-treatment EBV frequency greater than VRF 1E-7 had significantly higher risk of disease progression at three years (HR 1.8, CI 1.0-3.4, p=0.013) (Fig 1D).
Immunosuppression in transplant patients is associated with the expansion of the endogenous anellovirus family (De Vlaminck Cell 2013). Accordingly, anellovirus was detected significantly more often in PTLD patients (91% of samples) compared to DLBCL NOS (2.8%) and controls (1.4%) (Fig 1E, p < 0.0001). As the standard-of-care R-CHOP regimen for DLBCL has activity against both B- and T- lymphocytes, we hypothesized that an immunosuppressive effect might be observed. In non-transplant DLBCL patients receiving R-CHOP (n=31), we detected anellovirus in 6% of samples at the time of first chemotherapy infusion, 16% immediately before cycle 2, but in no samples from post-treatment patients in complete response (Fig 1F).
Viral integration into the host genome is associated with malignant transformation. We profiled a cohort of EBER+ cHL (n=8) and found circulating EBV/human chimeric reads suggesting integration in all cases. Viral fragment size distribution also distinguishes integrated DNA from shorter free episomes and may increase cancer screening performance (Lam PNAS 2018). We profiled EBV fragment sizes in cHL and PTLD patients grouped by EBER positivity. Plasma from EBER+ cHL and PTLD patients was significantly enriched in longer fragments (Fig 1G), suggesting nucleosomal protection of EBV integrated within tumor genomes but not their benign episomal counterparts.
Conclusions: Viral infection in lymphoma has diagnostic and prognostic significance: elevated circulating EBV levels are associated with active PTLD (Kanakry Blood 2016) and poor outcomes in advanced HL (Kanakry Blood 2013) and DLBCL (Tisi Leuk & Lymph 2015). Our work demonstrates the utility of cfDNA sequencing for simultaneous characterization of malignancy, infection, and immunosuppression. The integration of viral dynamics into cfDNA analysis may assist in risk stratification and treatment monitoring in lymphoma patients.
Dührsen:Amgen: Research Funding; Celgene: Honoraria, Research Funding; AbbVie: Consultancy, Honoraria; Roche: Honoraria, Research Funding; Gilead: Consultancy, Honoraria; Janssen: Honoraria. Hüttmann:Celgene: Other: Travel expenses; Roche: Other: Travel expenses. Meignan:F. Hoffman-La Roche Ltd: Honoraria. Casasnovas:Janssen: Consultancy; Takeda: Honoraria; Janssen: Honoraria; MSD: Honoraria; Merck: Honoraria; Gilead Sciences: Honoraria; Celgene: Honoraria; Roche: Consultancy; Roche: Research Funding; takeda: Consultancy; Gilead Sciences: Consultancy; Roche: Honoraria; Gilead Sciences: Research Funding; merck: Consultancy; MSD: Consultancy. Westin:Kite Pharma: Membership on an entity's Board of Directors or advisory committees; Apotex: Membership on an entity's Board of Directors or advisory committees; Novartis Pharmaceuticals Corporation: Membership on an entity's Board of Directors or advisory committees; Celgen: Membership on an entity's Board of Directors or advisory committees. Gaidano:Amgen: Consultancy, Honoraria; Morphosys: Honoraria; Janssen: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria; Roche: Consultancy, Honoraria. Advani:Bayer: Membership on an entity's Board of Directors or advisory committees, Other: Participated in an advisory board; Agensys: Research Funding; Infinity: Research Funding; Roche/Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Participated in an advisory board, Research Funding; Merck: Research Funding; Janssen: Research Funding; Cell Medica: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Participated in an advisory board; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Astra Zeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Participated in an advisory board; Seattle Genetics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Participated in an advisory board, Research Funding; Kyowa: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Participated in an advisory board; Pharmacyclics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Millenium: Research Funding; Celgene: Research Funding; Kura: Research Funding; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees, Other: Participated in an advisory board, Research Funding; Regeneron: Research Funding; Autolus: Membership on an entity's Board of Directors or advisory committees, Other: Participated in an advisory board; Gilead/Kite: Membership on an entity's Board of Directors or advisory committees, Other: Participated in an advisory board; Forty Seven Inc.: Research Funding; Celgene: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal